WHAT WE DO
Research
The future of precision oncology hinges on a profound comprehension of the intricate mechanisms underlying cancer progression and treatment resistance. In this pursuit, our research group is dedicated to unraveling the behavior of individual tumor cells, delving into the dynamics of cellular states and their cell-fate responses. By understanding these complexities, we aim to reveal crucial insights that can be applied to identify treatment-sensitive states of tumor cells and optimal treatment schedules.
Our primary focus is on cancer research, particularly emphasizing innovative early translational strategies for future treatments. We
specialize in both experimental and computational methodologies, including techniques such as long-term live single-cell measurements and understanding complex signaling dynamics.
A unique aspect of our work involves harnessing computational expertise for in-silico methods. This enables us to use mathematical and computational models to simulate the intricate processes of cancer evolution and signaling. This approach helps us better understand and predict responses to treatments, guiding our experiments and efficiently avoiding unnecessary animal testing while optimizing resource utilization.
The Granada Lab is committed to developing quantitative tools that comprehensively unveil the sources of heterogeneity within cancer, both temporally and spatially. Our broader objective is to contribute meaningfully to the understanding of cancer as a whole. A significant portion of our research is dedicated to addressing pressing biological questions within the context of cancer, with a specific emphasis on triple-negative breast cancer (TNBC). TNBC, affecting 15-20% of all breast cancer patients, stands out as one of the most heterogeneous and aggressive subtypes, characterized by high mortality rates. The urgent need for effective treatments is underscored by the current inability to predict the most suitable treatment for individual TNBC patients.
Situated within the Charité Comprehensive Cancer Center (CCCC) in Berlin, Germany, our lab actively engages in multidisciplinary collaborations to leverage our expertise. We aspire to make impactful contributions to the exploration of cancer, with a vision of advancing early translational research for the benefit of patients.
Single-cell cell-fate decisions
As part of the current standard treatment, most breast cancer patients receive a combination of chemotherapy and radiotherapy. Despite its success, still many patients gain little or no benefit from this treatment, as evidenced from the elevated rates of locoregional recurrence, distant metastatic spread, and cancer deaths. Unfortunately, those patients will nevertheless suffer the short and long-term side effects of the inefficient therapy. We aim to develop patient-specific strategies that maximize the damage in tumor cells while minimizing the damage to normal cells.
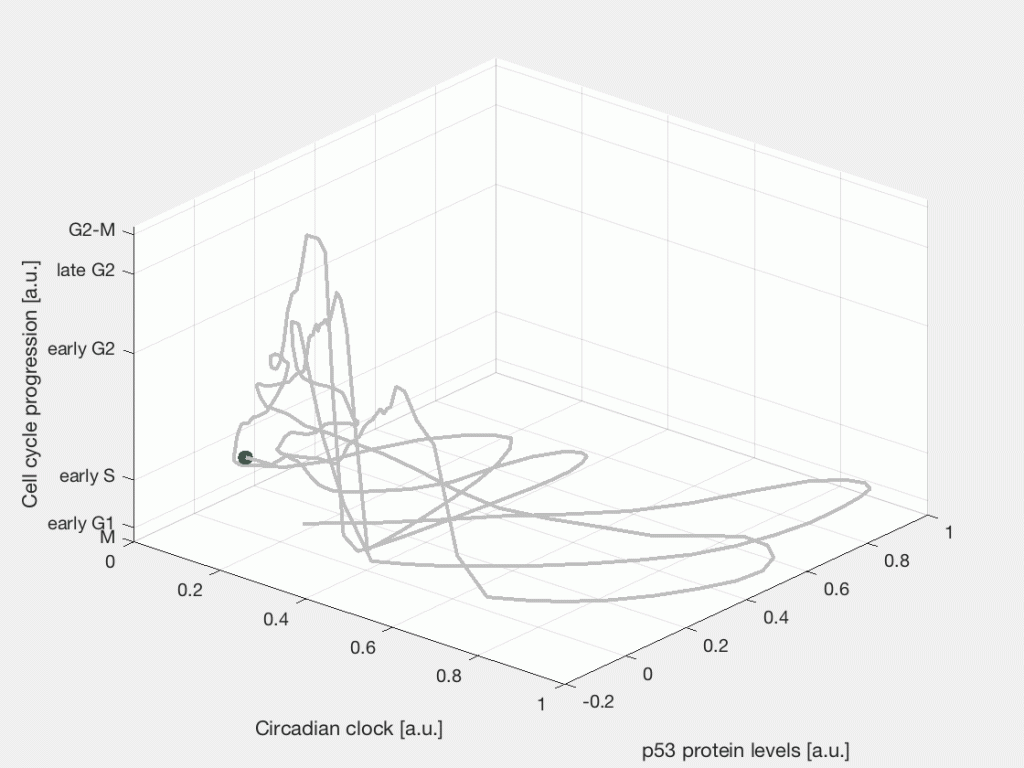
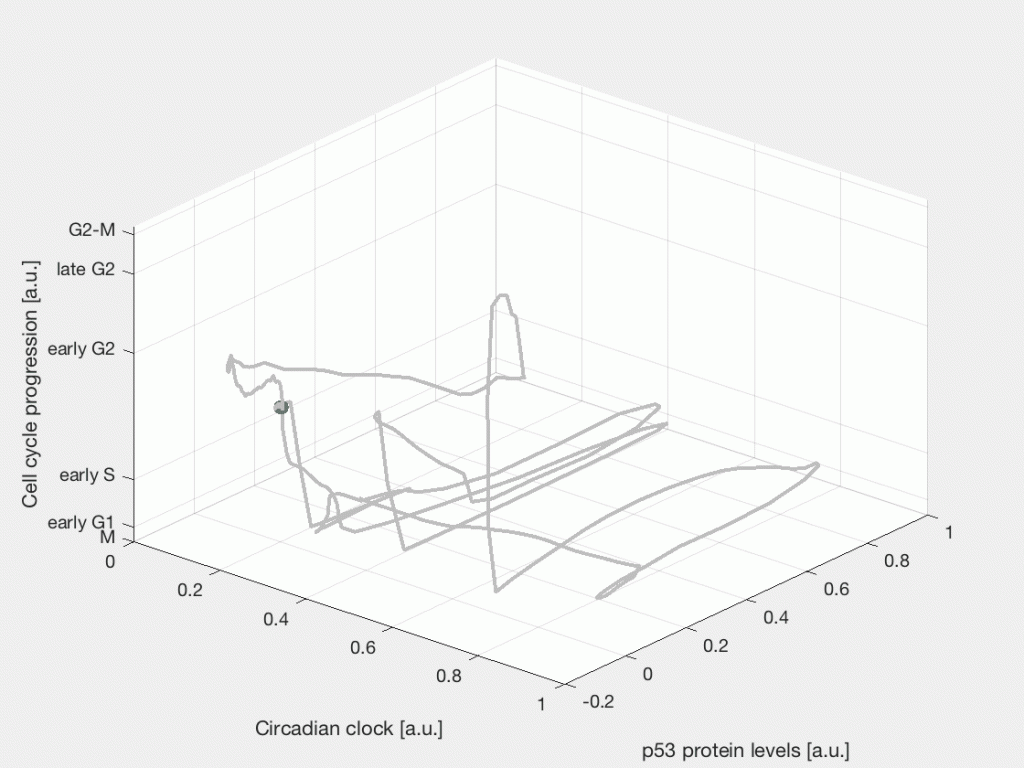
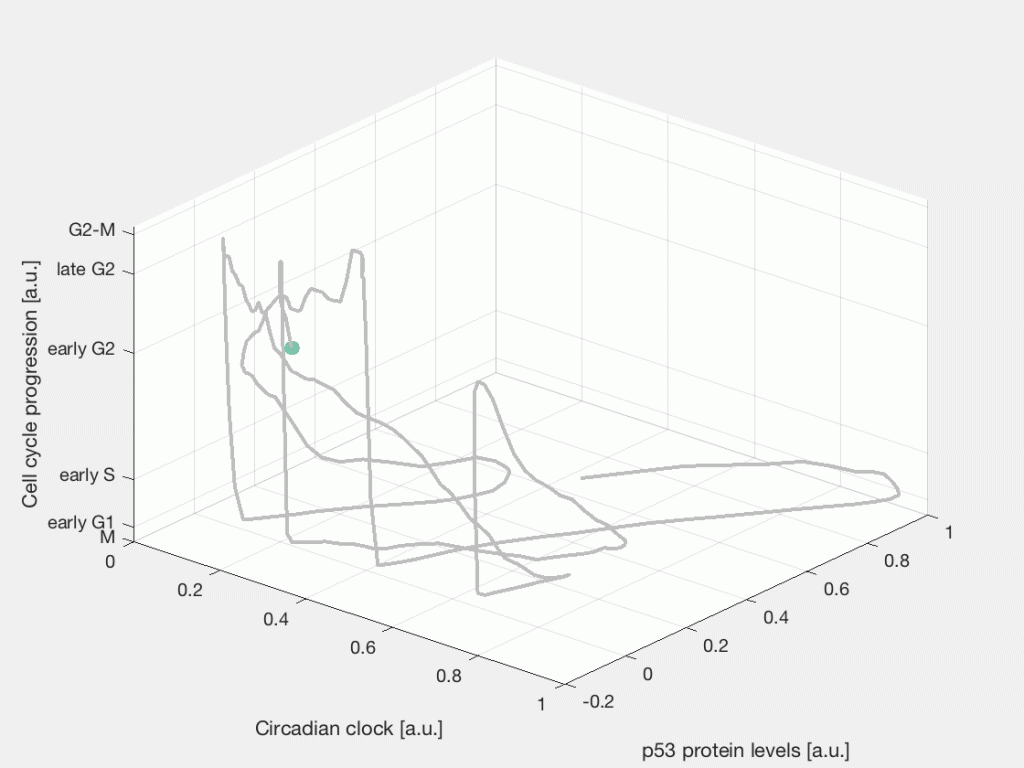
After the chemo- or radiotherapy a fraction of tumor cells will die, some cells will remain quiescent, while other cells will survive and continue dividing. This is a characteristic response to cancer therapy known as fractional response, where a subpopulation of cells survives the treatment. Part of our research focuses on studying the cellular events and signaling dynamics that control the heterogeneous cell fate response of individual cells in time. Specifically, how internal cycling factors, such as the circadian clock, the cell cycle, and the p53 protein dynamics interact with the DNA damage response pathway to determine the response to radiotherapy and chemotherapy in cancer cells.
Single-cell state space representation of three internal cellular variables
Proliferating breast cancer cells with p53 and 53BP1 markes
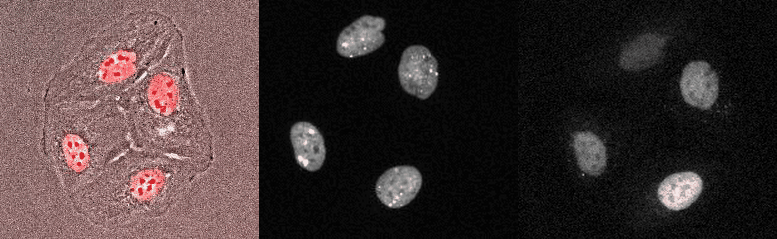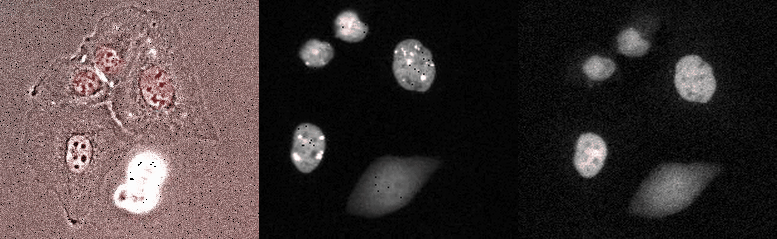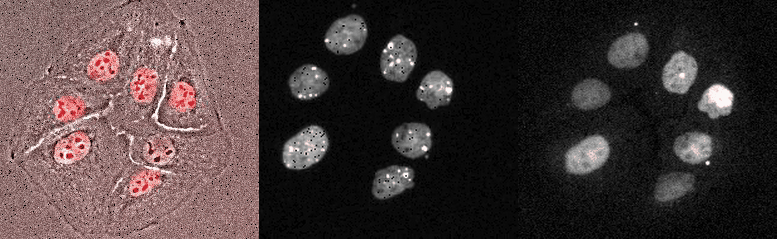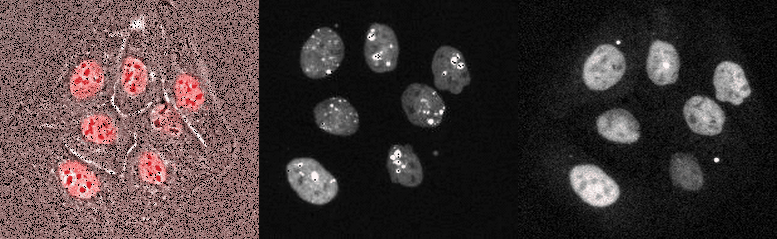
Mammalian Circadian Clock - from single cells to human behaviour
Single-cell circadian rhythms of well-synchronized SCN neurons.
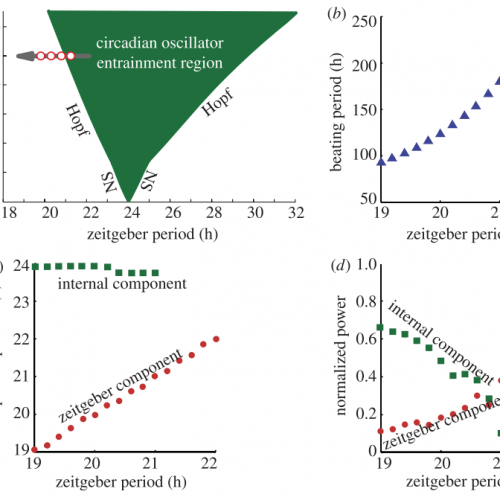
Intracellular Coupling and Entrainment Mechanisms
In addition to the SCN, peripheral oscillators, such as lung tissue, exhibit damped and usually less precise oscillations, which are thought to be brought about by the lack of intercellular coupling. Both SCN and oscillators in peripheral tissues share almost identical single-cell molecular clocks, but they behave surprisingly different upon external periodic perturbations (entrainment). Carrying out a combined theoretical and experimental study of whole tissue and single-cell circadian oscillations, my work has contributed to the understanding of the central role played by the intercellular coupling that explains the entrainment differences. In a separate theoretical study we focused on the different dynamics from hypothesized models of intercellular coupling. Our predictions clarified how coupling mechanisms affect entrainment properties.
- Abraham U*, Granada AE*, Westermark PO*, Heine M*, Kramer A, and Herzel H (2010) Coupling governs entrainment range of circadian clocks. Molecular Systems Biology, 6:438.
- Bordyugov G*, Granada AE*, and Herzel H (2011). How Coupling Determines the Entrainment of Circadian Clocks. European Physical Journal B.
- Erzberger A, Hampp G, Granada AE, Albrecht U, Herzel H (2013). Genetic redundancy strengthens the circadian clock leading to a narrow entrainment range. Journal of the Royal Society Interface 10(84):20130221.
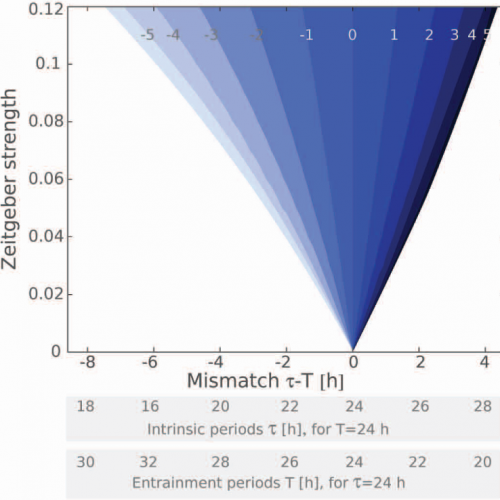
Circadian desynchronization and Timescales of entrainment
Experiments elucidating SCN heterogeneity are often invasive and thus implicitly modify the SCN tissue in an unknown manner. An novel experimental protocol was proposed to non-invasively dissociate this circadian oscillatory network in vivo. Rats exposed to exotic short Light-Dark cycles express two stable circadian motor activity rhythms (a fast and a slow rhythm). Motivated by these exciting works that characterized SCN heterogeneity, we developed a computational model and made predictions to help elucidate the sources of the observed heterogeneity (see first publication below). In addition, when an oscillator is entrained, its endogenous period is adjusted to that of the external recurring environment. Despite its heterogeneity, the SCN has the striking ability of fast entrainment. We have identified the core oscillator properties that determine the timescales to entrainment.
- Granada AE, Cambras T, Diez-Noguera A, Herzel H (2011). Circadian desynchronization. Journal of the Royal Society Interface 1:153-166.
- Granada AE, Herzel H (2009) How to achieve fast entrainment? The timescale to synchronization. PLoS One 4: e7057.
- Generic properties derived from the theory of coupled oscillators explain the observed differences in the timescales of entraiment.
Human Chronotypes and Seasonal effects
Commonly known as the “early birds” or “night owls”, a person’s chronotype is the propensity for the individual to sleep at a particular time during the day. A central question in human circadian research is to understand the interaction of the internal and environmental components that determine this chronotypes. Multiple major studies have characterized human chronotypes and study the distributions of human chronotypes but little is known about the factors that determine the shape of this distributions. In a series of works we have provided a framework to explain the distributions observed in human chronotypes and how the wake up time (phase of entrainment) depends on seasons and on additional internal human circadian clock parameters such as relaxation times and inter-cellular coupling.
- Granada AE, Bordyugov G, Kramer A, Herzel H (2013). Human chronotypes from a theoretical perspective. PLoS One 8(3):e59464.
- Granada AE, Herzel H, Kramer A, Abraham U. (2017). Information Transfer in the Mammalian Circadian Clock. Book chapter. Information and Communication Theory in Molecular Biology. Springer. ISBN-13: 978-3319547282
Perturbation and Information theory to study circadian oscillators.
Phase response curves are widely used in circadian clocks, neuroscience and heart physiology. They quantify the response of an oscillator to pulse-like perturbations. Phase response curves provide valuable information on the properties of oscillators and their synchronization. In the first publication listed below we discuss biological self-sustained oscillators (circadian clock, physiological rhythms, etc.) in the context of nonlinear dynamics theory. While direct synchronization by light is restricted to light-sensitive clock cells (e.g. in the eye), temperature cycles can be perceived by the majority of body cells, rendering it an elegant means to study environmental information transfer in mammalian clock cells. We studied the role of temperature oscillations as a zeitgeber for peripheral tissues. We combined information theory and theory of coupled oscillators to generate a set of theoretical predictions and tested them experimentally. See book chapeter below.
- Granada AE, Hennig RM, Ronacher B, Kramer A, Herzel H (2009) Phase response curves elucidating the dynamics of coupled oscillators. Methods in Enzymology 454:1–27.
- Granada AE, Herzel H, Kramer A, Abraham U. (2017). Information Transfer in the Mammalian Circadian Clock. Book chapter. Information and Communication Theory in Molecular Biology. Springer. ISBN-13: 978-3319547282
These works are done in collaboration with the Labs of Hanspeter Herzel and Achim Kramer, from Humboldt University Berlin and the Charité University Hospital, respectively.
Birdsong production
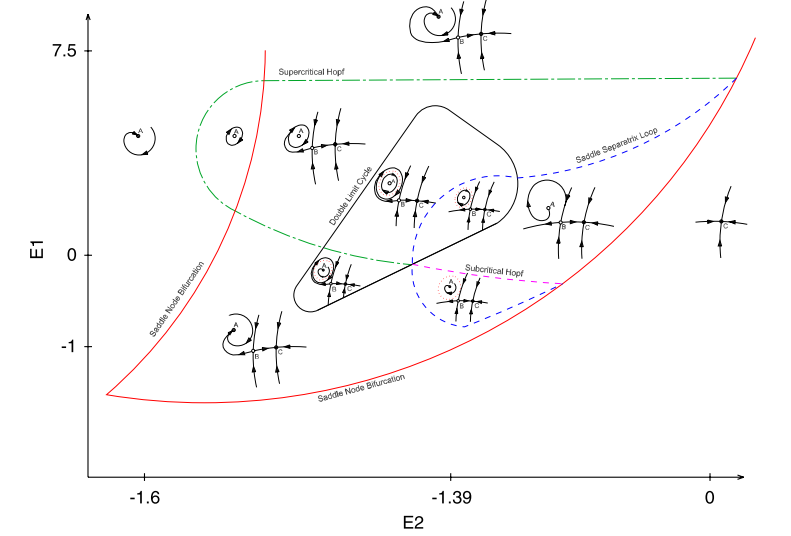
The generation of a behavior involves interactions between the nervous system, the morphology of the peripheral system and the environment. The biomechanics of a peripheral system imposes constraints on the neural control, and also provides opportunities for the emergence of complexity in behavior. A rich example is birdsong, where neural instructions drive a complex respiratory system in order to activate the vocal organ. The dynamical state of the respiratory system feeds back into the nuclei in charge of expiration and inspiration, and therefore the emerging dynamics can be potentially extremely rich. As part of my master thesis I use bifucation theory and simulations to inspected the respiratory patterns that can be generated as the result of the interaction of the respiratory nuclei and the respiratory peripheral system.
- Granada AE, Gabitto M, García G, Alliende J, Méndez J, Trevisan M, Mindlin GB (2006). The generation of respiratory rhythms in birds. Physica A. 371:84–87..